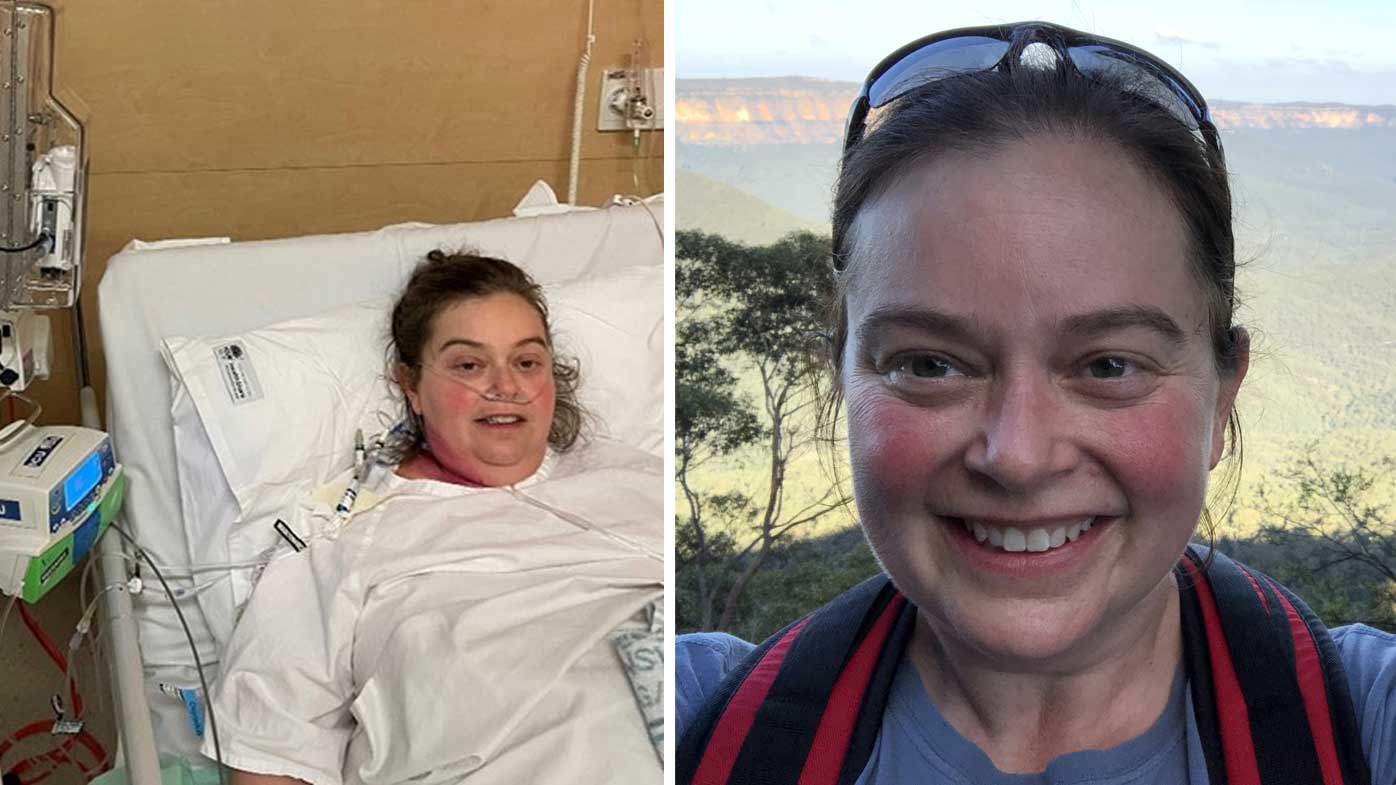
Jenny Abraham’s world shrank during the pandemic, but it wasn’t just because of the rolling lockdowns.
The Sydney woman had been living with pericarditis, an inflammatory condition affecting tissue around the heart, for around six years.
What had been a relatively manageable condition suddenly escalated after Abraham, who also has an autoimmune condition, contracted COVID-19 twice and suffered severe reactions to the vaccines.
“Every two to three days, I’d be having bouts of nine-out-of-10 chest pain. I would be on the floor struggling to breathe,” she said.
Abraham was told by doctors she should limit her movement to keep her heart rate down, but even walking around her apartment tired her out.
“You find yourself very quickly in a position where you’re not able to do a whole lot, and you’re in pain a lot of the time,” she said.
“It was a pretty awful time. The box I could operate in was getting smaller and smaller.”
Abraham had grown resistant to the steroid medications she was taking to try and control her pericarditis.
Knowing how serious her situation was, her immunologist mentioned there was one medication which could help her – Anakinra.
However, the drug is not listed on the Pharmaceutical Benefits Scheme (PBS) for pericarditis and costs $1200 a month.
READ MORE: ‘Incredible’: How mass pager explosion in Lebanon could have happened
Anakinra is included on the PBS for arthritis, but Australia is yet to follow the US and Europe in subsidising the drug for pericarditis.
While Abraham baulked at the cost, she knew she had to try it.
The effect, when she began taking the medication a year ago, was almost immediate and “life-changing”, she said.
“Within three days, I had no more pericarditis, it was gone,” she said.
“I was at the point where I was thinking about not being able to continue working. I’m in my mid to late 40s, so that’s a pretty awful outcome.
“Now, I’ve got back to doing photography, hiking, scuba diving – all the things I used to do before I got sick.”
Abraham said she was fortunate that, after three months of paying for the medication, her doctors helped her apply for the drug to be supplied to her through her hospital’s discretionary drugs fund.
However, the 12 months worth of funding would soon be reviewed.
“It’s turned back the clock 10 years on my disease, the thought of having to give that up is just horrifying,” she said.
Abraham said she was surprised at how difficult it was to access the medication when it was subsided overseas for the same condition.
“It’s incredibly frustrating that there are medicines that work out there which most people just can’t access because they are too expensive,” she said.
READ MORE: Why 1.9 billion people are obsessed with Pesto, the giant king penguin chick at a Melbourne aquarium
Australia lags behind US, Europe
It takes an average of 466 days for a medication approved by the Therapeutic Goods Administration (TGA) to be made available on the PBS, according to Medicines Australia.
Medicines Australia, which represents the research-based pharmaceutical industry, is today launching a campaign calling on that wait time to be cut to just 60 days.
“The way in which medicines are assessed for inclusion on the pharmaceutical benefits scheme has not kept pace with advances in research and innovation,” Medicines Australia CEO Liz de Somer said.
“Hundreds of thousands of Australians are missing out on access to the best medicine available for their condition and their circumstances because they aren’t available on the PBS.
“These delays are simply too long, and people are dying waiting.”
A Medicines Australia report from 2022 showed that between 2016-2021 Australia ranked 11th out of 20 OECD countries when it came to the average wait time for new medicines to be subsidised.
Japan boasted the lowest wait time, at 102 days, followed by Germany at 136 days and Austria at 153.
A recent government review found the system for acquiring medicines is too complex and patients are suffering the negative consequences of waiting to access new treatments.
Last week, the final report of the review into the Health Technology Assessment – which provides advice to the government on funding decisions – was handed down, including 50 recommendations for how to cut the time in which new treatments are made available and affordable.
The Albanese government has pledged to form an implementation group to review and carry out its recommendations.
De Somer, who was industry’s representative on the HTA Review Reference Committee, said the recommendations would make a significant difference to Australians once implemented as a complete package.
However, patient advocacy group Better Access Australia said while it supported the review’s aspiration to end the pricing games between pharmaceutical companies and the government, it believed the recommendations were focused too much on cost-cutting and not on patient delays.
“Savings are important and supported by Better Access Australia but they were not the primary objective of patients going into this review, and yet they will be the primary outcome,” Better Access Australia Chair, Felicity McNeill, said in a statement.
Better Access Australia is calling for a 100-day target for new medicines to be added to the PBS, saying the report does not go far enough.
“With best effort targets of 180-365 days for access to a few highly select new pharmaceutical listings under this report, the majority of medicines, devices and diagnostics will still be a two to five years wait,” McNeill said.
McNeill said the five-year wait for the shingles vaccine Shingrix to be funded was an example of the extreme delays patients are experiencing.
Do you have a story? Contact reporter Emily McPherson at emcpherson@nine.com.au
links to content on ABC
9News





