Counterfeit Ozempic-labelled pens thought to contain a different medication have been caught being imported into Australia, prompting an urgent warning from the Therapeutic Goods Administration (TGA).
“These pens may pose a serious health risk and should not be used,” the TGA said in a statement.
While Ozempic pens contain the drug semaglutide, used for treating diabetes and more recently for weight management, the counterfeit pens appear to be re-labelled insulin pens.
READ MORE: Rare virus with 88 per cent fatality rate emerges
“Insulin is a different type of medicine that should only be used when directed and prescribed by a health professional,” the TGA statement warned.
“Unintended use of insulin can cause dangerously low life-threatening blood sugar levels.”
One Australian who used a counterfeit semaglutide pen that they purchased overseas has already experienced a “life-threatening” reaction, according to the TGA.
Demand for Ozempic and other semaglutide medications has ballooned in recent years due to the drug’s weight loss effects.
From today, compounding pharmacies will be banned from producing replicas of semaglutide after a number of adverse reactions were reported, heightening a nationwide shortage and fuelling the black market trade.
READ MORE: Hundreds of stores to close as retail giant axes brands
The recent shipment of fake Ozempic products seized by the Australian Border Force was purchased online from an overseas website.
The pens are currently undergoing laboratory testing but the TGA warns that “they may pose a serious health risk and should not be used”.
Consumers are being urged to check their Ozempic medications for signs they could be counterfeit.
These include spelling errors, instruction leaflets not in English, unsealed packaging and changes in medicine size, shape and appearance.
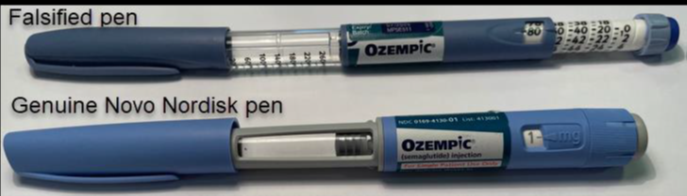
Both the imported counterfeit pens and those obtained overseas by the Australian patient had end caps that were blue, not grey.
The dosage barrels are in a different place, the stickers are not adhering properly to the pens and the rendering of text on the packaging is poor quality.
The batch numbers labelled on the pens – NPSG234 detected by the ABF and JS7A925 from the adverse event – have been confirmed as not genuine batch numbers by Novo Nordisk, the manufacturer of Ozempic.
“Consumers should be warned that manufacturers of counterfeit goods are producing products that, to the untrained eye, may appear legitimate, highlighting the need to purchase your medicines from legitimate sources,” the TGA said.
While importing semaglutide is legal so long as you have a valid prescription, importing counterfeit products – whether bought knowingly or unknowingly – is illegal under all circumstances.
links to content on ABC
9News





